Yes. This is standard practice among emergency medical personnel.

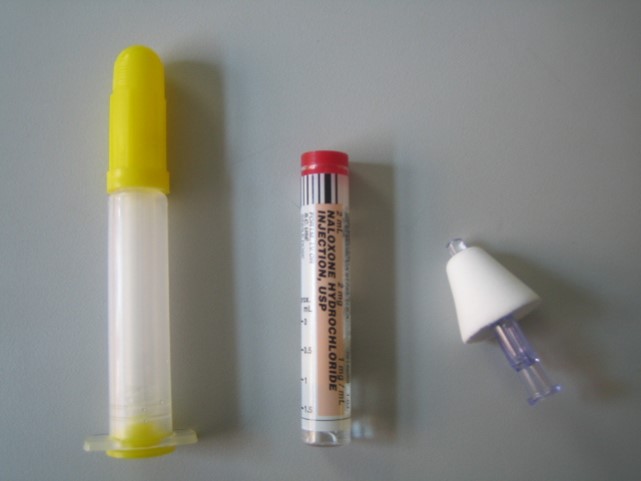

Law enforcement have three ways in which to administer naloxone. Currently, the most common administration method for law enforcement overdose rescue programs is intranasally (IN). During this mode of administration, a liquid form of naloxone is sprayed into the victim’s nostrils. Many first responders prefer IN delivery because it does not involve needles, eliminating the risk of an accidental needle stick injury. Naloxone is FDA-approved as an injectable drug and approved by the FDA through IN delivery in November 2015. Boston Basic Life Support medical responders have been using IN naloxone since 2005 to make IN doses available prior to FDA-approval. The needleless syringe containing the naloxone vial is first connected to a separate device called an atomizer that converts the liquid stream of the drug into a fine mist. The different components are typically sold separately, although limited quantities of pre-packaged IN rescue kits may be available from regional compounding pharmacies. The above does not pertain to the Adapt product which is a preloaded and ready for immediate use device
One alternative way to deliver naloxone is through intramuscular (IM) injection. This is an FDA-approved delivery process, utilized for decades in medical settings. Many community-based opioid overdose prevention programs distribute IM naloxone to families and other potential opioid overdose bystanders because of its lower cost. Although IN administration is far more common, several law enforcement agencies have opted for injectable administration. With IM, naloxone is drawn from a vial into a syringe, and then injected into the victim’s thigh or another large muscle.
Also available to the naloxone arsenal is a naloxone auto-injector product called EVZIO®. This device was the first naloxone product specifically FDA approved for administration for suspected opioid overdose outside a medically-supervised setting. It is designed to guide the user through the process of overdose reversal using pre-recorded audio prompts and printed images on the device displaying the administration steps. It injects naloxone utilizing a fully retractable needle system that is designed to eliminate the risk of needle stick injury. EVZIO® has a retail price that is substantially higher than the IN or IM products but the manufacturer, Kaléo, offers a program to make the product available at a discount to law enforcement agencies.
Approved by the FDA since the 1970s, naloxone is a very safe medication with the potential side effect of a theoretical risk of allergy that has never been documented. Its administration may result in acute opioid withdrawal (agitation, nausea, vomiting, diarrhea, "goose flesh", tearing, runny nose, and yawning). When victims experience these symptoms, they may become irritable and anxious. It is uncommon, however, for the revived victim to become violent or combative. Intranasal naloxone delivery is less likely to result in severe withdrawal symptoms than an injection.
On rare occasions, reviving an opioid overdose victim may restart existing health problems or uncover the effect of other drugs the victim had taken. This may result in heart palpitations or seizures. In all cases of overdose, it is critical victims be transferred to the care of medical professionals.
Your state health department or oversight agency may have an established data collection protocol so that they can evaluate the impact of the naloxone program. A few sample data collections forms can be found below, each of which can be adapted to suit your agency’s needs.